▲Introduction/Features
About 90% of patients receiving opioid pain control medication have experienced constipation, at present, the most frequent way to relieve constipation is the standard laxatives in the clinical hospice care. However, standard laxatives have worse therapeutic effects to OIC and more than 50% of patients’ demand for medical care is failed to be satisfied.
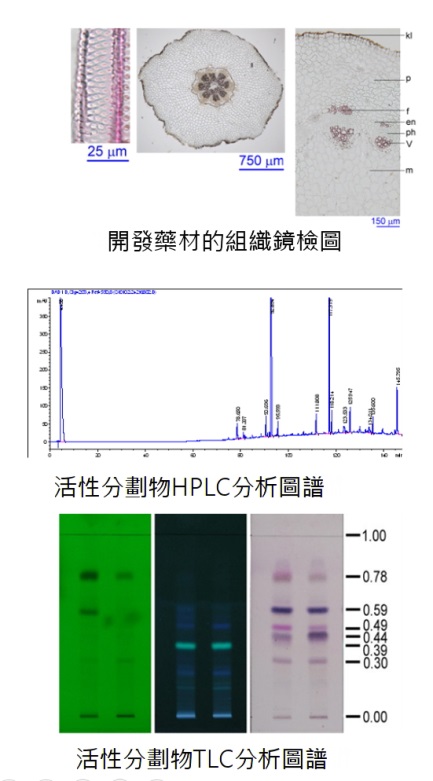
The research and development team of the botanicals of Medical and Pharmaceutical Industry Technology and Development Center is to evaluate in vivo efficacy of the single and compound prescriptions and select the OIC-treatable botanicals. The development of botanical products acquiring for industry needs is via several times and lots of drug activity comparisons and validations, efficacy increasing by improving extraction processes, safety assurance, and by medication specification and quality analytic technology establishment.
▲Technical advantages
Therapeutic medication originates from the natural and single medicine. Establish the specifications and quality analytic technology for medications and active fractions, and the manufacturing process meets the requirements for industry.
▲Patent management
1.Opioid pain control medication- the U.S./ China/ Taiwan.
2.Extend morphine pain control effect- Taiwan.
▲Scope of application
1.Relieve the side effect from cancer therapy-induced constipations and elevate quality of life for patients.
2.Solve the problem of methadone-induced constipations and increase the compliance for methadone treatment.
Finally, we develop safe and effective medicines with patients' acceptances to solve clinical factors like poor drug efficacy, inconvenient drug using, etc.