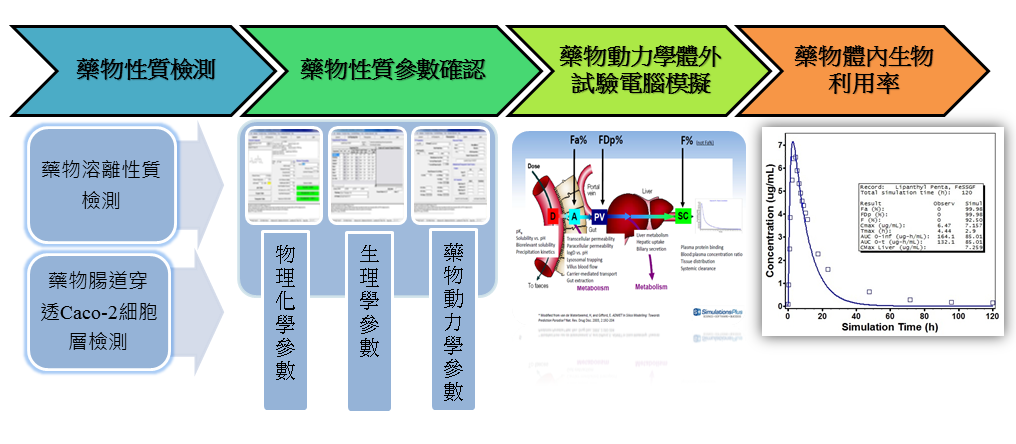
《Computer simulated dynamic in vitro testing》
▲Introduction/characteristics
In 2016, FDA and EMA had presented the guidance of the physiologically based pharmacokinetic (PBPK) modeling. In vitro PBPK computer simulation can provide the PBPK modeling information for the pharmaceutical industry, including investigational new drug (IND), new drug application (NDA), biologics license application (BLA), abbreviated new drug application (ANDA). Simulation in vivo absorption results will assist in vivo drug bioavailability prediction which further elevate the ingredient screening efficiency and bioequivalence (BE) successful rate.